Abstract
Introduction: Social determinants of health such as race, ethnicity, and socioeconomic status (SES) are associated with inferior outcomes in patients with B-cell acute lymphoblastic leukemia (B-ALL). Latinx patients have worse event-free survival and are at increased risk of relapse when compared to non-Latinx peers; given that low SES is also an independent predictor of relapse, some of this disparity is likely the result of structural racism. Chimeric antigen receptor T-cell therapy (CAR-T) is a promising approach to improve survival for patients with relapsed/refractory (r/r) B-ALL. However, given the limited number of cellular therapy centers, these therapies may not be equally accessible to patients of low SES or patients from historically disadvantaged populations. The sociodemographic characteristics of pediatric and young adult patients referred for CAR-T have not been well-described. We aimed to evaluate how sociodemographic characteristics of patients referred for CAR-T from outside institutions (referred CAR-T) differed from r/r patients referred for CAR-T at their home institution (local CAR-T), r/r patients not referred for CAR-T at their home institution (other relapse), and patients without r/r disease (without relapse). We hypothesized that there would be a higher proportion of non-Latinx White patients and patients with higher SES among referred CAR-T patients.
Methods: We conducted a multicenter, retrospective cohort study of children and young adults with B-ALL treated at five large pediatric hospitals between 2012-2018. Patients diagnosed with B-ALL between age 0 and 29 were included. Clinical and demographic data (including race/ethnicity, insurance, and primary language) were collected from the electronic health record and analyzed using descriptive statistics. For patients residing within the United States, ArcGIS NSES Index software was used to assign SES score by census tract (0-100, with 50 as the national average).
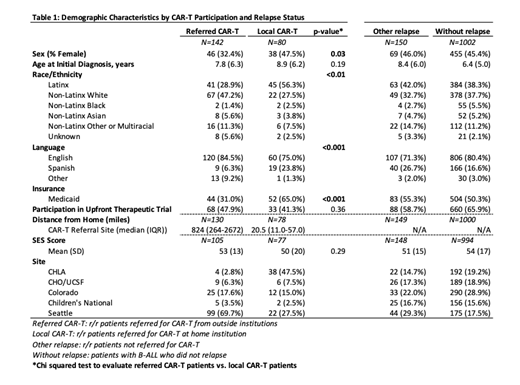
Results: Our cohort included 1374 patients with B-ALL, of which 372 (27%) had r/r disease (Table 1). Among 372 r/r patients, 142 were referred CAR-T patients and 230 were treated for r/r leukemia at their home institution (35% local CAR-T and 65% other relapse). Median distance traveled to CAR-T therapy was 824 miles among referred patients and 20.5 miles among local CAR-T patients. The majority of patients (76% of the overall cohort and 79% of those receiving CAR-T) were either White or Latinx.
A higher proportion of local CAR-T patients were Latinx compared to referred CAR-T patients (56% vs. 29%; p<0.01). Conversely, fewer local CAR-T patients were non-Latinx White compared to referred CAR-T patients (28% vs. 47%; p<0.01). A higher proportion of local CAR-T patients had public insurance compared to referred CAR-T patients (65% vs. 31%; p<0.001). A higher proportion of local CAR-T patients identified Spanish as their primary language compared to referred CAR-T patients (24% vs. 6%; p<0.001). Mean SES scores were similar across all groups.
Conclusions: In a large multicenter cohort of B-ALL pediatric and young adult patients, the demographics of referred CAR-T patients were notably different than local CAR-T patients. Latinx patients are at increased risk of relapse, making access to CAR-T all the more essential; however, we found less than one-third of patients referred from outside institutions for CAR-T cell therapy were Latinx, while a majority of patients receiving CAR-T cell therapy locally were Latinx. This association suggests that barriers to access such as distance and need for travel may differentially impact Latinx patients due to structural racism. Spanish-speaking patients and patients with public insurance were also underrepresented in referrals from outside institutions. However, these observational differences may be in part due to regional variations in the demographics of the population. Preliminary analysis by site is underway and indicates that center level demographic variation may be driving some of the observed differences. Very few Black and Asian patients received CAR-T within our cohort making any assessments of patterns of care difficult. Future work will involve additional analysis to identify non-clinical factors that may influence referral and enrollment rates on CAR-T clinical trials as well as qualitative work to understand these factors from the patient and provider perspectives.
Walters: AllCells, Inc: Consultancy; BioLabs, Inc: Consultancy; Vertex pharmaceuticals: Consultancy; Ensoma, Inc.: Consultancy. Ramsey: Vertex Pharmaceuticals: Consultancy; Cystetics Medicines: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal